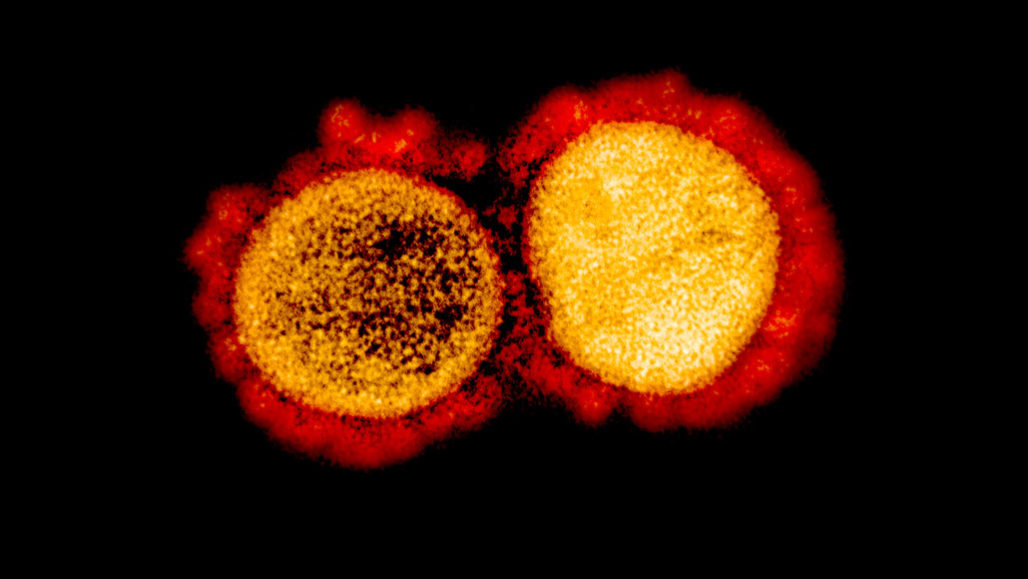
With the novel coronavirus, COVID-19 is spreading across the world and the fact that no drug or treatment has been found against it is creating fear among people. Coronavirus is a large family of enveloped, single-stranded RNA that infects mainly mammals including humans. In humans, coronavirus causes mild to severe respiratory illness. These viruses exhibit strong virulence and are highly contagious. While a person infected, produces mild symptoms, certain individuals respond severely, sometimes leading to death.
The SARS pandemic, back in 2002 is known to belong to the same family of viruses as COVID-19. According to WHO, SARS rapidly spread through 29 countries, with 8096 confirmed cases, but the current pandemic has surpassed all numbers by infecting over millions of people across the world. Today, it is due to SARS that has resulted in a coordinated effort to develop treatments targeting the virus or host cell components responsible for viral replication.
The deadly virus, COVID-19 enters the human body and binds to the target cells through angiotensin-converting enzyme 2 (ACE2) which is mainly expressed in endothelial cells and Leydig cells. ACE-2, a transmembrane metallo carboxypeptidase, is an enzyme which for years has been important for the treatment of hypertension. With further Polymerase Chain Reaction (PCR) analysis it was determined that the ACE-2 receptor is also present in the lung and gastrointestinal tract, tissues harboring COVID-19.
Inhibiting ACE2 receptor blocks COVID-19 entry:
ACE-2 belonging to the family of ACE receptors, plays a key role in the Renin-Angiotensin System (RAS) and in the treatment of hypertension. ACE2 is known to degrade angiotensin II and thereby, negatively regulating RAS. Recently experts have revealed that the COVID-19 virus uses ACE-2 receptors as their entry in HeLa cells. Additionally, it was found that by using anti- ACE-2 antibodies in other mammals like monkeys, it was seen that there was an entry blockage of pseudotypes expressing the COVID-19 virus.
For the virus to enter the host cell, its spike glycoprotein (S) needs to be cleaved at 2 sites, termed S protein primming so the viral and host cells membrane can fuse. A serine protease TMPRSS2 is essential for cleaving the S protein. It was found by treating Calu-3 human lung cell line with a serine inhibitor- camostat mesylate the entry of the COVID-19 virus was partially blocked. Angiotensin-converting enzyme (ACE) inhibitors is also found to play a role in preventing the formation of angiotensin II. ACE multifaceted functions include treating heart failure, controlling high blood pressure, and preventing kidney failure in diabetic patients.
Existing concerns about ACE inhibitors:
Though ACE inhibitors might seem like a promising solution for COVID-19, there are certain concerns regarding the increased susceptibility to COVID-19. These are considered based on the fact that ACE inhibitors are also used in treating millions of people with hypertension, heart, and kidney disease. When administered with inhibitors, diabetes and hypertension patients were observed to have an increase in ACE2, which in turn would facilitate infection with COVID-19. It is hence hypothesized that treating diabetic and hypertension patients with ACE inhibitors might make them more susceptible to COVID-19.
Furthermore, several studies have reported that long term usage of ACE inhibitors can modify the adaptive immune response, which is a key and much-needed defense against any infection. These particular effects must be taken into noticed and investigated further in context to COVID-19.
Road to COVID-19 therapies:
These findings could greatly impact the efforts being taken in developing treatments for the current pandemic. For instance, TMPRSS2 inhibitors can be potentially used to prevent virus entry into the host cells. Though there are certain drawbacks in using ACE inhibitors, there is a lack of scientific evidence and clinical data to support the discontinuing of ACE inhibitors in COVID-19 patients with existing hypertension and diabetes. The proof that reduction in mortality due to ACE inhibitors and the beneficial effects outweigh the theoretical risks.
Our interpretation of this hypothesis should not lead to changing drugs for patients with diabetes or hypertension, without consulting an expert physician. Though it’s of utmost urgency for the scientific community to come up with some solution for this deadly virus, further research and clinical trials need to be performed before any of the said theory can become a reality.