Small Cell Lung Cancer (SSLC) is a very particular lethal malignant cancer type for which effective therapies are urgently needed. Small cell carcinomas are usually centrally located, arising from the bronchus, with a small number of peripheral lesions. They obstruct most of the airways through circumferential compression.
Around 15% of lung cancers are classified under SCLC. Recent studies indicate that SCLC can be split into 4 major subtypes, based on the expression YAP1, ASCL1, NEUROD1, or POU2F3. Treatments for these subtypes are not yet standardized, due to the lack of information on their physiology.
Now scientists have summarised their new findings “MYC Drives Temporal Evolution of Small Cell Lung Cancer subtypes by reprogramming Neuroendocrine Fate” in Cancer Cell about the physiology of SCLC subtypes, which could potentially pave the way to study and treat the disease.
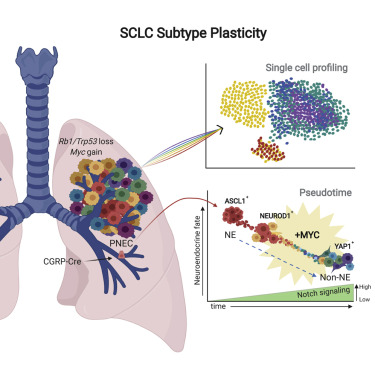
Historically SCLC has been treated as a single disease. The disease exhibits genetic loss of tumor suppressors along with the expression of MYC, MYCL, or MYCN. Large scale gene expression analyses suggest that SCLC subtypes have distinct vulnerabilities which need to be understood to improve treatment. Subtype SCLC-ASCL1 which comprises 70% of the tumors is a regulator of neuroendocrine (NE) fate. Using genetically engineered mouse models (GEMM) it was found that ASCL1 is important for tumor development and that MYCL was highly expressed in this subtype. Contrastingly the other SCLC subtypes constituting 30% found to overexpress MYC and exciting low non-NE cell fate. Research shows that Myc expression drives a non-NE SCLC in GEMMs, however, the relationship between the subtypes SCLC-A and ACLC-N and involvement of MYC in driving other subtypes is still unknown.
In this paper, the author and his team investigate MYC origins and its relationship with the SCLC subtypes. Functional data from the study suggest that MYC is behind the evolution of SCLC subtypes. In context to the times’ suppressor loss, MYC promotes temporal evolution from SCLC-A to SCLC-N to SCLC-Y in-vivo. The results from the study also revealed that MYC does not work alone and requires the help of the NOTCH signaling pathway to drive tumor progression.
The study further revealed that both MYCL and MYC are not functionally redundant in SCLC. They correlate with distinct gene expression and localize to super-enhancers. MYC and MYCL have the ability to change a cell morphology fate, molecular subtype, and influence drug sensitivity.
Different SCLC subtypes inhibit different targeting drugs and considering this, dynamically evolving tumors are termed as moving therapeutic targets. Over the years many targeted therapies have failed to treat SCLC like chemotherapy. The authors of this study speculate that chemotherapy has remained the most effective due to its non-specificity among subtypes that evolve. The findings from these studies demonstrate that SCLC and other similar cancer types would benefit from a combination or customized therapies.